America met Zantac in 1995, in the midst of growing stomach acid issues and demand for relief. What 15 million Americans taking prescription doses (and millions more taking over-the-counter amounts) of Zantac per year didn’t know, was that almost three decades later, the FDA would launch an investigation that would end Zantac’s era for good…and raise concern nationwide.
Like all recalled drugs, it’s been realized that people are at high risk for suffering consequences due to Zantac. If you have suffered from Zantac injuries, you may be eligible for compensation through a Zantac lawsuit.
What is Zantac?
Zantac is the American-owned brand name for ranitidine, a stomach acid reducer belonging to a larger group of drugs called histamine H2-receptor antagonists. Prescription Zantac is used to heal or avert ulcers, gastroesophageal reflux disease, and other severe heartburn conditions related to stomach acid build-up. Over-the-counter Zantac helps to reduce/prevent heartburn, acid indigestion, and an upset stomach.
Why Was Zantac Taken Off the Market?
What was once a drug sold in colossal amounts meant to help people, ended up being what hurt people. Zantac’s cancer-causing properties were discovered by routine testing of medications, and later correlated with an alarming number of rare cancers forming in users.
Pharmaceutical companies like Valisure found that a known carcinogen, N-Nitrosodimethylamine (NDMA), was able to form in ranitidine in extremely high and dangerous amounts both over time and when stored at high temperatures. Ranitidine was found to be simply unstable, meaning the NDMA levels were not due to contamination of the medicine. Ranitidine breaks down to a carcinogen on its own.
For perspective, NDMA is so potent with cancerous properties that it is commonly used by scientists as a control to induce cancer in animals in clinical studies. Valisure reported that one pill of Ranitidine (or Zantac) can convert to 3mL of NDMA in the body. This is an extreme cause for concern and the discovery launched a full investigation by the FDA in 2019.
By April 2020, Zantac (and all other ranitidine products both prescribed and OTC) were pulled from the shelves. What’s most upsetting for victims of Zantac is the ongoing investigation of doctors and producers of Zantac, for the fact that they may have known of Zantac’s cancerous potential and put the drug on the market anyways.
Zantac Alternatives
If you’re prone to ulcers, heartburn, or a variety of stomach acid-induced problems, searching for a new form of alleviation is a matter of grave concern. The FDA and other health organizations have made some drug suggestions that will provide the same relief intended, without the carcinogenic risk. This classification of drugs is called proton pump inhibitors (or PPIs):
1. Esomeprazole (Brand Name: Nexium)
Nexium’s main function is to block acid production that is used to treat stomach and duodenal ulcers, GERD, and Zollinger-Ellison syndrome.
2. Lansoprazole (Brand Name: Prevacid)
Prevacid is very similar to Nexium, with a few discrepancies. Prevacid prevents stomach and intestinal ulcers, erosive damage to the esophagus, and stomach acid imbalances like Zollinger-Ellison syndrome.
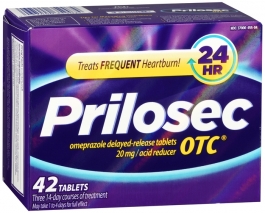
3. Omeprazole (Brand Name: Prilosec)
Prilosec is very similar to Nexium and may be prescribed based on the individual doctor’s preference between the two. Some studies have concluded that Prilosec works faster, and other studies have concluded that the effects and response times of the medications are similar. Prilosec treats heartburn, ulcers, GERD, and Zollinger-Ellison syndrome.
Regardless of the medication, the FDA has only identified the presence of NDMA in ranitidine and nizatidine. You are safe to take any remaining PPIs for relief, and entitled to compensation for any Zantac suffering you may have gone/are going through. Consult with a lawyer if you believe you’ve been affected by Zantac.







No Comment